I meant strictly as a kinetic energy release from binding energy.
Okay. Well, as you know, each fission of a U-235 nucleus results in a release of about 210Mev of energy, about 80-85 percent of which is kinetic energy of the fission fragments. The remainder is in the form of gammas, neutron energies, and other subatomic particles.
How do we get 210MeV, however? Simple--Einstein showed us that matter does equate energy, to be specific, 931MeV per amu. If we take the mass of the individual components (neutrons + protons), we would find that their mass is greater than the measured mass of the nucleus. This mass defect per nucleon is referred to as the binding energy, and can be shown in a curve which looks something like this:
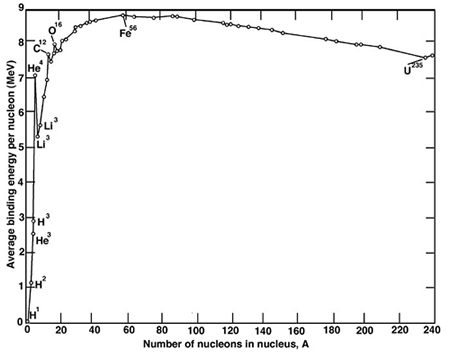
So if we take the average mass of the fission fragments and neutrons, and compare them to the mass of the neutron plus Uranium nucleus, the difference in mass is about 0.22 amu, or 210 Mev.